Embark on a journey into the realm of gravimetric analysis lab AP chemistry, where precision and accuracy converge to unravel the mysteries of matter. This in-depth exploration unveils the fundamental principles, diverse applications, and advanced techniques that empower chemists in various fields to quantitatively determine the mass of analytes with unparalleled precision.
Delve into the intricacies of gravimetric analysis, uncovering the methods, factors, and strategies that govern its accuracy. Discover the art of data analysis and interpretation, unlocking the secrets hidden within experimental results. Prepare to be captivated by the transformative power of gravimetric analysis, a cornerstone of analytical chemistry.
Introduction to Gravimetric Analysis in AP Chemistry: Gravimetric Analysis Lab Ap Chemistry

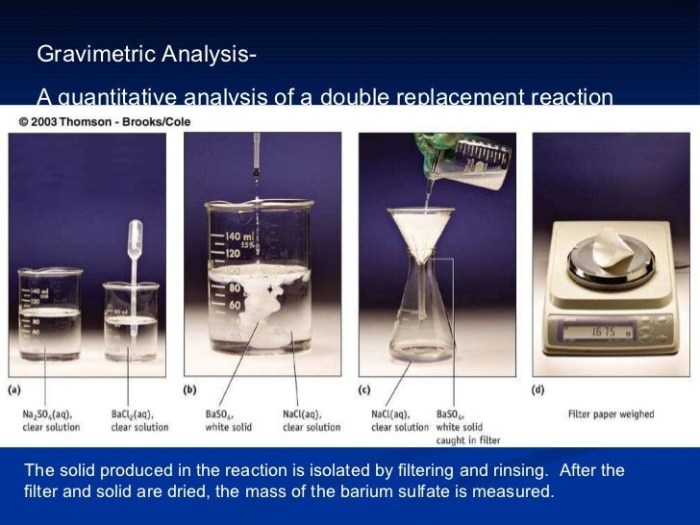
Gravimetric analysis is a quantitative analytical technique used to determine the mass of a specific analyte in a sample. It involves separating the analyte from the sample, converting it into a form of known composition, and measuring its mass. Gravimetric analysis finds applications in various fields of chemistry, including AP Chemistry, where it is commonly used to determine the purity of compounds, analyze the composition of mixtures, and quantify the concentration of ions in solutions.
Common Methods in Gravimetric Analysis
There are several methods employed in gravimetric analysis, each with its advantages and disadvantages. The most common methods include:
- Precipitation:This method involves adding a reagent to the sample, causing the analyte to precipitate out of solution as an insoluble solid. The precipitate is then filtered, washed, dried, and weighed to determine the mass of the analyte.
- Evaporation:In this method, the sample is evaporated to dryness, leaving behind the analyte as a solid residue. The residue is then weighed to determine the mass of the analyte.
- Ignition:This method involves heating the sample to a high temperature, causing the analyte to decompose and volatilize. The remaining residue is then weighed to determine the mass of the analyte.
Factors Affecting Gravimetric Analysis Accuracy
The accuracy of gravimetric analysis can be affected by several factors, including:
- Purity of reagents:Impurities in the reagents used can introduce errors into the analysis.
- Environmental conditions:Factors such as temperature and humidity can affect the precipitation, evaporation, and ignition processes.
- Equipment calibration:Improperly calibrated equipment can lead to inaccurate measurements.
- Human error:Mistakes in weighing, filtering, and other procedures can affect the results.
To minimize these factors, it is essential to use high-quality reagents, control environmental conditions, calibrate equipment regularly, and follow proper laboratory procedures.
Techniques for Enhancing Gravimetric Analysis, Gravimetric analysis lab ap chemistry
Several advanced techniques can be used to improve the accuracy and precision of gravimetric analysis, including:
- Microgravimetric analysis:This technique uses specialized equipment to measure the mass of very small samples.
- Thermogravimetric analysis:This technique measures the mass of a sample as it is heated or cooled, providing information about its thermal stability and composition.
- Electrogravimetric analysis:This technique involves depositing the analyte onto an electrode by electrolysis, allowing for precise determination of its mass.
These techniques offer advantages such as increased sensitivity, reduced sample size, and improved accuracy.
Data Analysis and Interpretation in Gravimetric Analysis
Data obtained from gravimetric analysis is analyzed to determine the mass of the analyte. Statistical techniques, such as calculating the mean and standard deviation, are used to evaluate the reliability of the results. The mass of the analyte is then used to calculate its concentration in the sample.
Drawing meaningful conclusions from experimental data requires considering the accuracy and precision of the analysis, as well as any potential sources of error. By carefully interpreting the data, chemists can gain valuable insights into the composition and properties of the sample.
Q&A
What is the fundamental principle of gravimetric analysis?
Gravimetric analysis relies on the quantitative determination of an analyte’s mass by converting it into a compound of known composition and accurately measuring its mass.
What are the advantages of gravimetric analysis?
Gravimetric analysis offers high accuracy and precision, minimal susceptibility to interferences, and the ability to analyze a wide range of analytes.
What factors can affect the accuracy of gravimetric analysis?
Factors such as reagent purity, environmental conditions, equipment calibration, and human error can influence the accuracy of gravimetric analysis.
What are some advanced techniques used to enhance gravimetric analysis?
Advanced techniques like microgravimetric analysis, thermogravimetric analysis, and electrogravimetric analysis improve the accuracy and precision of gravimetric analysis.